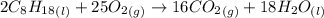Chemistry, 26.08.2019 21:00 ilovebeans25423
The combustion of octane, c8h18, proceeds according to the reaction
2c8h18(l)+25o2(> 16co2(g)+18h2o(l)
if 297 mol of octane combusts, what volume of carbon dioxide is produced at 20.0 °c and 0.995 atm?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

You know the right answer?
The combustion of octane, c8h18, proceeds according to the reaction
2c8h18(l)+25o2(> 16co2(...
2c8h18(l)+25o2(> 16co2(...
Questions

Mathematics, 21.09.2020 01:01

Geography, 21.09.2020 01:01

English, 21.09.2020 01:01



English, 21.09.2020 01:01



Computers and Technology, 21.09.2020 01:01


English, 21.09.2020 01:01


Social Studies, 21.09.2020 01:01




Mathematics, 21.09.2020 01:01