
Chemistry, 29.01.2020 04:49 kimlyn58p0wyn0
If a given power plant released so2 gas with a volume v of 1200 m3 at a density ρ of 2.86 kg/m3 at standard pressure and temperature, how many moles n of so2 are released? the atomic weight of sulfur is 32.07 u and the atomic weight of oxygen is 16.00 u.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 22.06.2019 20:00
Listenbase your answer to the question on the information below.nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. an external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body.cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. one hospital keeps a 100.0-gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment.which choice represents the correct product for the beta decay of the co-60? fe-60ni-60fe-61ni-61
Answers: 2


Chemistry, 23.06.2019 09:00
Chortling is used to clean water. another possible atom that would also work is a. sodium b. sulfur c. bromine
Answers: 1
You know the right answer?
If a given power plant released so2 gas with a volume v of 1200 m3 at a density ρ of 2.86 kg/m3 at s...
Questions


English, 16.10.2020 14:01

English, 16.10.2020 14:01

Chemistry, 16.10.2020 14:01




Social Studies, 16.10.2020 14:01


Mathematics, 16.10.2020 14:01





English, 16.10.2020 14:01

Chemistry, 16.10.2020 14:01



Physics, 16.10.2020 14:01

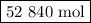
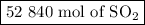 .
.


