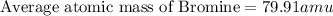Chemistry, 03.02.2020 01:53 graceaowen
1.
a student looked up the naturally occurring isotopes of bromine and found the following information:
50.54% of the naturally occurring isotopes of bromine have an atomic mass of 78.92 amu while 49.46% of the
naturally occurring isotopes of bromine have an atomic mass of 80.92 amu. calculate the average atomic mass of
bromine. show all work

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3

Chemistry, 23.06.2019 01:00
Who examines and coordinates the cleanup of polluted sites?
Answers: 2
You know the right answer?
1.
a student looked up the naturally occurring isotopes of bromine and found the following info...
a student looked up the naturally occurring isotopes of bromine and found the following info...
Questions

English, 21.11.2019 15:31

History, 21.11.2019 15:31

Chemistry, 21.11.2019 15:31



World Languages, 21.11.2019 15:31



Mathematics, 21.11.2019 15:31


Social Studies, 21.11.2019 15:31


History, 21.11.2019 15:31

Mathematics, 21.11.2019 15:31



Mathematics, 21.11.2019 15:31


Mathematics, 21.11.2019 15:31

History, 21.11.2019 15:31

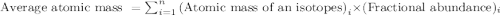 .....(1)
.....(1)![\text{Average atomic mass of Bromine}=[(78.92\times 0.5054)+(80.92\times 0.4946)]](/tpl/images/0494/7606/3343d.png)