
Chemistry, 04.02.2020 20:45 tyliyahmiles99
Calculate delta h in kj for the following reactions using heats of formation:
a) 2c2h6 (g) + 7o2 (g) > 4co2 (g) +6h2o (g)
b) 2pbo (s) + pbo2 (s) > pb3o4 (s)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1
You know the right answer?
Calculate delta h in kj for the following reactions using heats of formation:
a) 2c2h6 (g) +...
a) 2c2h6 (g) +...
Questions


English, 28.08.2019 01:30

Mathematics, 28.08.2019 01:30



Arts, 28.08.2019 01:30






Arts, 28.08.2019 01:30

English, 28.08.2019 01:30



Chemistry, 28.08.2019 01:30

Physics, 28.08.2019 01:30



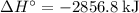 per mole reaction.
per mole reaction. per mole reaction.
per mole reaction.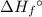 of a substance?
of a substance? 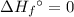 for the most stable allotrope of each element under standard conditions. For example, oxygen
for the most stable allotrope of each element under standard conditions. For example, oxygen 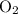 (not ozone
(not ozone 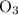 ) is the most stable allotrope of oxygen. Also, under STP
) is the most stable allotrope of oxygen. Also, under STP 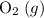 from itself does not involve any chemical or physical change. As a result,
from itself does not involve any chemical or physical change. As a result, 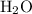 in particular) and the sign of the enthalpy changes.
in particular) and the sign of the enthalpy changes.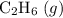 :
: 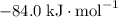 ;
;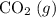 :
: 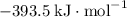 ;
;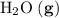 :
: 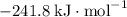 ;
;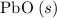 :
: 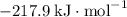 ;
;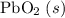 :
: 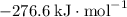 ;
;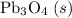 :
: 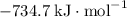
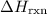 (or simply
(or simply 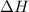 from enthalpies of formation?
from enthalpies of formation?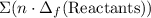 to show that this value takes the coefficients into account.Multiply the enthalpy of formation of each reactant by its coefficient in the equation.Find the sum of these values. Label the sum
to show that this value takes the coefficients into account.Multiply the enthalpy of formation of each reactant by its coefficient in the equation.Find the sum of these values. Label the sum 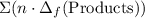 to show that this value takes the coefficient into account.Change = Final - Initial. So is the case with enthalpy changes.
to show that this value takes the coefficient into account.Change = Final - Initial. So is the case with enthalpy changes. 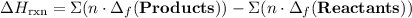 .
. 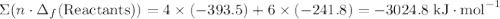 ;
;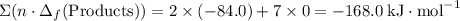 ;
;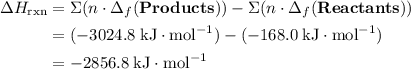 .
.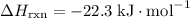 .
.

