
Which statement describes the potential energy diagram of an endothermic reaction?
a. the potential energy of the products is equal to the potential energy of the reactants.
b. the potential energy of the reactants is greater than the potential energy of the products.
c. the activation energy of the reactants is less than the activation energy of the products.
d. the potential energy of the products is greater than the potential energy of the reactants.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1


Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2
You know the right answer?
Which statement describes the potential energy diagram of an endothermic reaction?
a. the po...
a. the po...
Questions



English, 09.03.2021 18:10

Mathematics, 09.03.2021 18:10

Mathematics, 09.03.2021 18:10










Mathematics, 09.03.2021 18:10





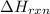 comes out to be positive.
comes out to be positive.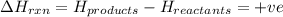
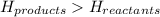 for endothermic reactions
for endothermic reactions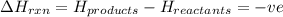
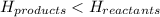 for exothermic reactions
for exothermic reactions


