
Chemistry, 21.12.2019 01:31 tayjohn9774
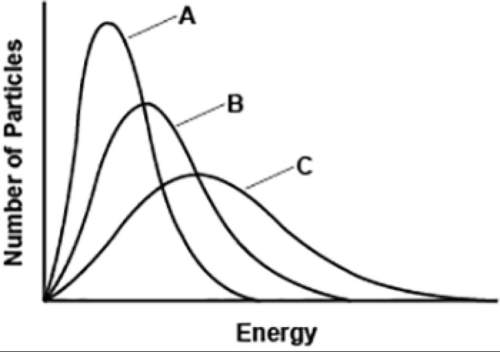
The graph shows a sample of gas when it is hot, cold, and at room temperature.
three graphs are plotted with number of particles on the y axis and energy on the x axis. all three graphs are smooth curves which rise up and then come down. the leftmost curve is the highest and is labeled a. the rightmost curve is labeled c and is the lowest. the curve in the middle is labeled b and has a height less than a and greater than c.
which statement is correct about curves b and c?
b represents hot gas and c represents gas at room temperature.
b represents gas at room temperature and c represents hot gas.
b represents gas at room temperature and c represents cold gas.
b represents cold gas and c represents gas at room temperature.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

You know the right answer?
The graph shows a sample of gas when it is hot, cold, and at room temperature.
three gra...
three gra...
Questions

History, 12.12.2020 16:40


Mathematics, 12.12.2020 16:40





English, 12.12.2020 16:40








Mathematics, 12.12.2020 16:40


Mathematics, 12.12.2020 16:40

Arts, 12.12.2020 16:40

World Languages, 12.12.2020 16:40



