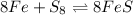Look at the following reaction. select the option that best applies to the reaction.
8fe + s8 ⇄ 8fes
eight moles of reactants form eight moles of product.
nine moles of reactants form eight moles of product.
eight moles of products form eight moles of reactants.
this reaction is not at equilibrium.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
List the two type of transporst that the cell in orde to transport molecules acroos the membrane
Answers: 1


Chemistry, 23.06.2019 01:30
In which phase of mitosis do the spindle fibers pull the chromosomes apart to opposite sides of the cell ?
Answers: 1

Chemistry, 23.06.2019 04:31
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1
You know the right answer?
Look at the following reaction. select the option that best applies to the reaction.
8fe + s8...
8fe + s8...
Questions



















Business, 18.10.2019 19:20