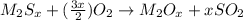Acompound contains a metal and sulfur. this compound goes through a combustion reaction such that compound x is produced from the metal atoms and compound y is produced from the sulfur atoms in the reactant. what are the compounds x and y?
a. x is carbon dioxide, and y is sulfur dioxide.
b. x is a hydrocarbon, and y is sulfur powder.
c. x is a metal oxide, and y is sulfur dioxide.
d. x is a metal hydride, and y is sulfur powder.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2
You know the right answer?
Acompound contains a metal and sulfur. this compound goes through a combustion reaction such that co...
Questions




History, 25.11.2021 14:00





Spanish, 25.11.2021 14:00


History, 25.11.2021 14:00



English, 25.11.2021 14:00

Mathematics, 25.11.2021 14:00


Social Studies, 25.11.2021 14:00

French, 25.11.2021 14:00


History, 25.11.2021 14:00