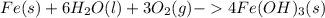One of the most recognizable corrosion reactions is the rusting of iron. rust is caused by iron reacting with oxygen gas in the presence of water to create an oxide layer. iron can form several different oxides, each having its own unique color. red rust is caused by the formation of iron(iii) oxide trihydrate. in the space provided, write the balanced reaction for the formation of fe2o3•3h2o(s). phases are optional.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2
You know the right answer?
One of the most recognizable corrosion reactions is the rusting of iron. rust is caused by iron reac...
Questions


Mathematics, 12.02.2021 19:30

Biology, 12.02.2021 19:30

English, 12.02.2021 19:30


Mathematics, 12.02.2021 19:30

Mathematics, 12.02.2021 19:30

Mathematics, 12.02.2021 19:30

Arts, 12.02.2021 19:30

Business, 12.02.2021 19:30


Mathematics, 12.02.2021 19:30

Mathematics, 12.02.2021 19:30

English, 12.02.2021 19:30

Mathematics, 12.02.2021 19:30

English, 12.02.2021 19:30



Health, 12.02.2021 19:30