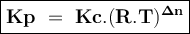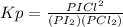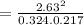Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:10
Which statement is true about the part of the electromagnetic spectrum that human eyes can detect? it contains only the colors of the rainbow and television waves. o it is divided into seven ranges of wavelengths. it contains ultraviolet, visible, and infrared light. it is divided into nine ranges of wavelengths.
Answers: 2

Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 21.06.2019 23:00
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1
You know the right answer?
Consider the reaction: i2(g)+cl2(g)⇌2icl(g) kp= 81.9 at 25 ∘c. calculate δgrxn for the reaction at...
Questions

Advanced Placement (AP), 23.08.2021 18:10

English, 23.08.2021 18:10


Mathematics, 23.08.2021 18:10

Mathematics, 23.08.2021 18:10






Mathematics, 23.08.2021 18:10

Mathematics, 23.08.2021 18:10

Chemistry, 23.08.2021 18:10

Biology, 23.08.2021 18:10

History, 23.08.2021 18:10



Mathematics, 23.08.2021 18:20


![\large {\boxed {\bold {Kc ~ = ~ \frac {[C] ^ m [D] ^ n} {[A] ^ p [B] ^ q}}}}](/tpl/images/0425/9888/55d71.png)
![\large {\boxed {\bold {Kp ~ = ~ \frac {[pC] ^ m [pD] ^ n} {[pA] ^ p [pB] ^ q}}}}](/tpl/images/0425/9888/1041d.png)