
Chemistry, 23.09.2019 15:30 babyrocks7300
If 30.0 ml of ca(oh)2 with an unknown concentration is neutralized by 40.0 ml of 0.175 m hcl, what is the concentration of the ca(oh)2 solution? show all of the work needed to solve this problem. (2 points) ca(oh)2 + 2hcl yields 2h2 o + cacl2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 23.06.2019 02:30
What role does weathering have in shaping earth’s surface? a) it allows sediments to fall out of a medium. b) it sediments settle on a new surface. c) it breaks down older material into sediments. d) it transports sediments to a different location. will give brainliest, answer quickly.
Answers: 2

Chemistry, 23.06.2019 03:30
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1

Chemistry, 23.06.2019 04:00
Silver reacts with oxygen to produce silver oxide. (write balanced chemical equation and identify type of chemical reaction.)
Answers: 1
You know the right answer?
If 30.0 ml of ca(oh)2 with an unknown concentration is neutralized by 40.0 ml of 0.175 m hcl, what i...
Questions

Social Studies, 31.10.2019 01:31


Social Studies, 31.10.2019 01:31


Mathematics, 31.10.2019 01:31

Mathematics, 31.10.2019 01:31


Mathematics, 31.10.2019 01:31





Chemistry, 31.10.2019 01:31



Physics, 31.10.2019 01:31

English, 31.10.2019 01:31

Biology, 31.10.2019 01:31


Biology, 31.10.2019 01:31

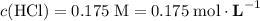 .
.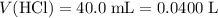 .
.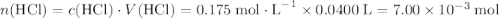 .
.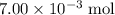 of HCl will neutralize only half that much Ca(OH)₂.
of HCl will neutralize only half that much Ca(OH)₂.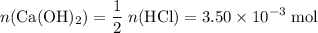 .
.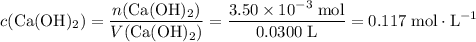 .
.

