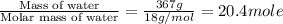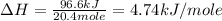Chemistry, 28.09.2019 22:00 emalvidrez5205
The initial temperature of the water in a constant-pressure calorimeter is 24°c. a reaction takes place in the calorimeter, and the temperature rises to 87°c. the calorimeter contains 367 g of water, which has a specific heat of 4.18 j/(g·°c). calculate the enthalpy change (δh) during this reaction

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 22.06.2019 23:00
Movement that is like a t a type of wave that transfers energy where the particles in the medium move in a circle motion while the energy travels left or right. a type of wave that transfers energy where the particles in the medium move perpendicular to the direction in which the energy is traveling. transfers energy from one location to another a type of wave that transfers energy where the particles in the medium move parallel to the direction in which the energy is traveling. movement that is back and forth, like an equal sign = 1. wave 2. parallel movement 3. perpendicular movement 4. transverse wave 5. longitudinal wave 6. surface wave
Answers: 1
You know the right answer?
The initial temperature of the water in a constant-pressure calorimeter is 24°c. a reaction takes pl...
Questions







Chemistry, 26.07.2019 03:30



English, 26.07.2019 03:30



History, 26.07.2019 03:30


Social Studies, 26.07.2019 03:30

Mathematics, 26.07.2019 03:30

Social Studies, 26.07.2019 03:30

Social Studies, 26.07.2019 03:30


Mathematics, 26.07.2019 03:30

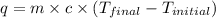
 = specific heat of water =
= specific heat of water = 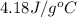
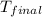 = final temperature of water =
= final temperature of water = 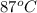
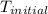 = initial temperature of metal =
= initial temperature of metal = 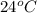
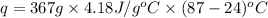
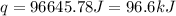
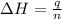
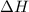 = enthalpy change = ?
= enthalpy change = ?