Read the given equation. 2na + 2h2o → 2naoh + h2
during a laboratory experiment, a certain qu...

Chemistry, 03.11.2019 07:31 twalters88
Read the given equation. 2na + 2h2o → 2naoh + h2
during a laboratory experiment, a certain quantity of sodium metal reacted with water to produce sodium hydroxide and hydrogen gas. what was the initial quantity of sodium metal used if 6.30 liters of h2 gas were produced at stp?
a.) 10.3 grams
b.) 12.9 grams
c.) 14.7 grams
d.) 15.2 grams

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1



Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
Questions


English, 03.03.2022 05:50


Business, 03.03.2022 05:50

Biology, 03.03.2022 05:50



Mathematics, 03.03.2022 05:50





Mathematics, 03.03.2022 05:50

Biology, 03.03.2022 05:50


Mathematics, 03.03.2022 05:50

History, 03.03.2022 05:50



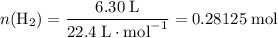 .
.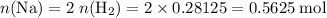 .
.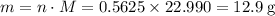 .
.

