
Chemistry, 28.01.2020 13:40 heyyyyy3922
As with any combustion reaction, the products of combusting a hydrocarbon fuel (cxhy) with oxygen (o2) are carbon dioxide (co2) and water (h2o).
a mass of 15.51 g for an unknown fuel was combusted in a reaction vessel containing an unknown amount of oxygen. at the end of the reaction, there still remained 15.46 g of the fuel as well as 0.0817 g of water and 0.1497 g of carbon dioxide. the oxygen was completely consumed during the reaction.
how many molecules of oxygen gas were initially present in the reaction vessel?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1


Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1
You know the right answer?
As with any combustion reaction, the products of combusting a hydrocarbon fuel (cxhy) with oxygen (o...
Questions

Computers and Technology, 08.11.2019 23:31


Biology, 08.11.2019 23:31

History, 08.11.2019 23:31

Chemistry, 08.11.2019 23:31

Mathematics, 08.11.2019 23:31

Mathematics, 08.11.2019 23:31

History, 08.11.2019 23:31


English, 08.11.2019 23:31

Geography, 08.11.2019 23:31




Mathematics, 08.11.2019 23:31

Mathematics, 08.11.2019 23:31



Mathematics, 08.11.2019 23:31

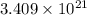
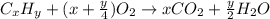
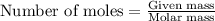
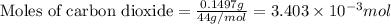
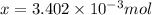
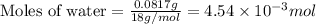
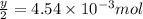
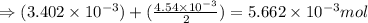
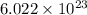 number of molecules.
number of molecules.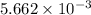 moles of oxygen will contain =
moles of oxygen will contain = 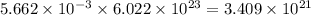 molecules.
molecules.

