
Chemistry, 26.12.2019 07:31 rileymorrison8825
What stress would shift the equilibrium position of the following system to the right?
n2o3(g) ⇌ no(g) + no2(g); δh is negative
decreasing the concentration of n2o3
heating the system
adding a catalyst
increasing the concentration of no

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 23:00
What is the mass of naoh that would have to be added to 500 ml of a solution of 0.20 m acetic acid in order to achieve a ph of 5.0?
Answers: 1

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2


Chemistry, 23.06.2019 18:40
Select the correct answer from each drop-down menu. in a reversible reaction, the forward reaction takes place the reverse reaction. such a reaction, on reaching equilibrium, will have .
Answers: 2
You know the right answer?
What stress would shift the equilibrium position of the following system to the right?
n2o3(g...
n2o3(g...
Questions



Chemistry, 15.06.2021 21:20

Biology, 15.06.2021 21:20

Physics, 15.06.2021 21:20



Mathematics, 15.06.2021 21:20




Mathematics, 15.06.2021 21:20


English, 15.06.2021 21:20

Mathematics, 15.06.2021 21:20

Mathematics, 15.06.2021 21:20

Mathematics, 15.06.2021 21:20



Mathematics, 15.06.2021 21:20

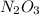 => To the left
=> To the left

