

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Zinc + lead(ii) nitrate yield zinc nitrate + leadwhat's the chemical equation for this?
Answers: 1

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1
You know the right answer?
Caco3(s) ∆→cao(s) + co2(g).
if 13.2 g of co2 was produced from the thermal decomposition of 40...
if 13.2 g of co2 was produced from the thermal decomposition of 40...
Questions


English, 19.05.2021 22:30

Mathematics, 19.05.2021 22:30




Computers and Technology, 19.05.2021 22:30

Mathematics, 19.05.2021 22:30

English, 19.05.2021 22:30









Mathematics, 19.05.2021 22:30


Physics, 19.05.2021 22:30

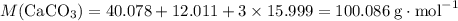 .
.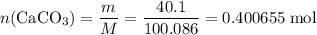 .
.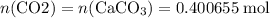 .
.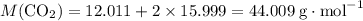 .
.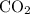 expected for the 40.1 grams of CaCO₃:
expected for the 40.1 grams of CaCO₃: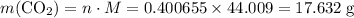 .
. .
.

