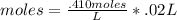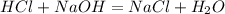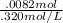Chemistry, 25.09.2019 06:50 vickReis06
Astudent titrated 20 ml of 0.410 m hcl with 0.320 m naoh. determine the volume of naoh needed at equivalence point

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:20
1. suppose a reaction mixture, when diluted with water, afforded 300 ml of an aqueous solution of 30 g of the reaction product malononitrile [ch2(cn)2], which is to be isolated by extraction with ether. the solubility of malononitrile in ether at room temperature is 20.0 g/100 ml, and in water is 13.3 g/100 ml. what weight of malononitrile would be recovered by extraction with (a) three 100-ml portions of ether and (b) one 300-ml portion of ether? suggestion: for each extraction, let x equal the weight extracted into the ether layer. in part (a), the concentration in the ether layer is x/100 and in the water layer is (30 x)/300; the ratio of these quantities is equal to k 20/13.3.
Answers: 2

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3
You know the right answer?
Astudent titrated 20 ml of 0.410 m hcl with 0.320 m naoh. determine the volume of naoh needed at equ...
Questions


Mathematics, 04.05.2021 01:40



Engineering, 04.05.2021 01:40


Advanced Placement (AP), 04.05.2021 01:40

Mathematics, 04.05.2021 01:40

History, 04.05.2021 01:40

English, 04.05.2021 01:40


Biology, 04.05.2021 01:40

Mathematics, 04.05.2021 01:40







Mathematics, 04.05.2021 01:40

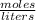 ) and the volume of the solution (.02L).
) and the volume of the solution (.02L).