Which of the following is true for the equilibrium constant of a reaction?
a. it is a ratio o...


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1


Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3
You know the right answer?
Questions

Mathematics, 09.04.2021 03:20

Biology, 09.04.2021 03:20

Mathematics, 09.04.2021 03:20


Mathematics, 09.04.2021 03:20



Mathematics, 09.04.2021 03:20


Mathematics, 09.04.2021 03:20


Mathematics, 09.04.2021 03:20

Computers and Technology, 09.04.2021 03:20


Computers and Technology, 09.04.2021 03:20

Mathematics, 09.04.2021 03:20

Chemistry, 09.04.2021 03:20



Mathematics, 09.04.2021 03:20

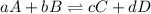
![K=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0433/4366/91a0a.png)


