
consider the substances below.
• 1 mol of beryllium
• 1 mol of salt
• 1 mol of water
• 1 mol of hydrogen
which statement is true about these substances? 1) they have exactly the same mass.2) they have different numbers of particles.3) they have the same number of atoms.4) they have different masses.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

You know the right answer?
consider the substances below.
• 1 mol of beryllium
• 1 mol of salt
• 1 mol of water<...
• 1 mol of beryllium
• 1 mol of salt
• 1 mol of water<...
Questions

Mathematics, 05.01.2020 11:31


English, 05.01.2020 11:31


Chemistry, 05.01.2020 11:31

Mathematics, 05.01.2020 11:31


Mathematics, 05.01.2020 11:31

Mathematics, 05.01.2020 11:31



Biology, 05.01.2020 11:31



Mathematics, 05.01.2020 11:31

Social Studies, 05.01.2020 11:31

History, 05.01.2020 11:31



Health, 05.01.2020 11:31

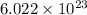 number of particles.
number of particles.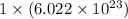 number of atoms.
number of atoms.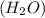 has 3 number of atoms. So, 1 mole of water contains
has 3 number of atoms. So, 1 mole of water contains 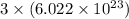 number of atoms.
number of atoms.

