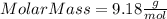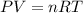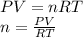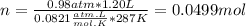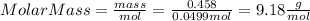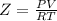Part 1. determine the molar mass of a 0.458-gram sample of gas having a volume of 1.20 l at 287 k and 0.980 atm. show your work. part 2. if this sample was placed under extreme pressure, describe how the actual volume would compare to the predicted volume. explain your answer.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3
You know the right answer?
Part 1. determine the molar mass of a 0.458-gram sample of gas having a volume of 1.20 l at 287 k an...
Questions

Biology, 28.08.2019 06:00


Mathematics, 28.08.2019 06:00



Mathematics, 28.08.2019 06:00




Biology, 28.08.2019 06:00

History, 28.08.2019 06:00



English, 28.08.2019 06:00

History, 28.08.2019 06:00

Mathematics, 28.08.2019 06:00

Mathematics, 28.08.2019 06:00

Mathematics, 28.08.2019 06:00

English, 28.08.2019 06:00