Chemistry, 03.02.2020 08:57 tasnimabdallah971
Energy and specific heat
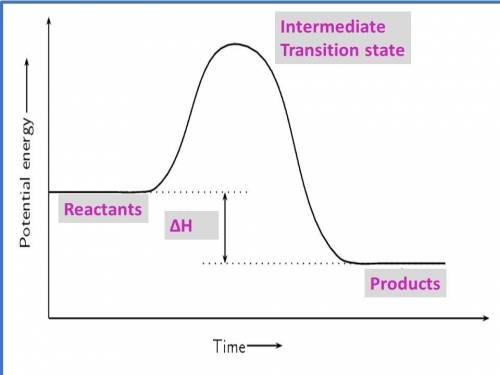
1. draw a graph of an exothermic reaction. label reactants, products and ∆h.
2. calculate the amount of energy required to raise the temperature of 3.00g of gold from 45.9 to 93.0°c.
3. 1.70g of a silvery metal requires 1000.j of energy to change its temp from 298k to 2749k. is the metal pure silver?


Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1

Chemistry, 23.06.2019 10:30
Amethod of separation that employs a system with two phases of matter, a mobile phase and a stationary phase, is called
Answers: 2

You know the right answer?
Energy and specific heat
1. draw a graph of an exothermic reaction. label reactants, prod...
1. draw a graph of an exothermic reaction. label reactants, prod...
Questions

Mathematics, 17.06.2020 23:57

Mathematics, 17.06.2020 23:57



Mathematics, 17.06.2020 23:57

Biology, 17.06.2020 23:57

Mathematics, 17.06.2020 23:57



Mathematics, 17.06.2020 23:57

Mathematics, 17.06.2020 23:57

Spanish, 17.06.2020 23:57

Mathematics, 17.06.2020 23:57

History, 17.06.2020 23:57


Social Studies, 17.06.2020 23:57


Mathematics, 17.06.2020 23:57

Physics, 17.06.2020 23:57

Mathematics, 17.06.2020 23:57