
Chemistry, 02.10.2019 01:00 divagothboi
During an exothermic reaction, δh for the reactants was 890 kj/mol. which of the following statements is correct about the δh for the products?
a. it is less than 890 kj/mol because the amount of energy required to break bonds is less than the amount of energy released in forming bonds.
b. it is less than 890 kj/mol because the amount of energy required to break bonds is greater than the amount of energy released in forming bonds.
c. it is greater than 890 kj/mol because the amount of energy required to break bonds is less than the amount of energy released in forming bonds.
d. it is greater than 890 kj/mol because the amount of energy required to break bonds is greater than the amount of energy released in forming bonds.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1


Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
During an exothermic reaction, δh for the reactants was 890 kj/mol. which of the following statement...
Questions

Mathematics, 26.10.2021 01:00

Arts, 26.10.2021 01:00



English, 26.10.2021 01:00

History, 26.10.2021 01:00

Biology, 26.10.2021 01:00




Computers and Technology, 26.10.2021 01:00


Social Studies, 26.10.2021 01:00







Health, 26.10.2021 01:00

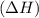 comes out to be positive.
comes out to be positive.

