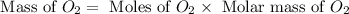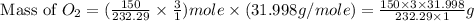Chemistry, 31.01.2020 09:02 gamingisfun
In an experiment, zinc chlorate decomposed according to the following chemical equation.
zn(clo3)2 → zncl2 + o2
(molar mass of zn(clo3)2 = 232.29 g/mol; zncl2 = 136.286 g/mol; o2 = 31.998 g/mol)
if the mass of zinc chlorate was 150 grams, which of the following calculations can be used to determine the mass of oxygen gas formed?
(150 × 1 × 232.29) ÷ (31.998 × 3) grams
(150 × 3 × 232.29) ÷ (31.998 × 1) grams
(150 × 1 × 31.998) ÷ (232.29 × 3) grams
(150 × 3 × 31.998) ÷ (232.29 × 1) grams

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1
You know the right answer?
In an experiment, zinc chlorate decomposed according to the following chemical equation.
...
...
Questions

Mathematics, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

English, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

English, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

Physics, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

History, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

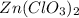
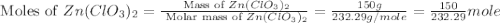
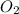
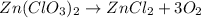
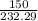 moles of
moles of 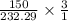 moles of
moles of