
Chemistry, 22.11.2019 06:31 myaaa13754
Find the concentration of h+ ions at a ph = 11 and
ph = 6. then divide the concentration of h+ ions at a
ph = 11 by the of h+ ions at a ph = 6. record your answer in table c.
what is the concentration of h+ ions at a ph = 11?
mol/l
what is the concentration of h+ ions at a ph = 6?
mol/l
how many fewer h+ ions are there in a solution at a
ph = 11 than in a solution at a ph = 6?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 23.06.2019 03:00
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1

Chemistry, 23.06.2019 03:30
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1
You know the right answer?
Find the concentration of h+ ions at a ph = 11 and
ph = 6. then divide the concentration of h+...
ph = 6. then divide the concentration of h+...
Questions



Social Studies, 19.08.2019 01:00



Chemistry, 19.08.2019 01:00


Chemistry, 19.08.2019 01:00

Mathematics, 19.08.2019 01:00


Social Studies, 19.08.2019 01:00

Social Studies, 19.08.2019 01:00

Mathematics, 19.08.2019 01:00


Mathematics, 19.08.2019 01:00


World Languages, 19.08.2019 01:00


Mathematics, 19.08.2019 01:00

Biology, 19.08.2019 01:00

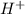 ion concentration.
ion concentration.![pH=-\log[H^+]](/tpl/images/0386/0640/cf945.png)
![11=-\log[H^+]](/tpl/images/0386/0640/c91a3.png)
![[H^+]=1\times 10^{-11} mol/L](/tpl/images/0386/0640/23467.png) ..(1)
..(1)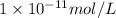 .
.![6=-\log[H^+]'](/tpl/images/0386/0640/502fa.png)
![[H^+]'=1\times 10^{-6} mol/L](/tpl/images/0386/0640/fdc73.png) ..(2)
..(2)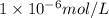 .
.![\frac{[H^+]}{[H^+]'}=\frac{1\times 10^{-11} mol/L}{1\times 10^{-6} mol/L}=1\times 10^{-5}](/tpl/images/0386/0640/2e79f.png)
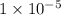 .
.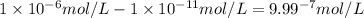
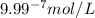 ions fewer than in a solution at a pH = 6
ions fewer than in a solution at a pH = 6

