
Chemistry, 13.11.2019 00:31 zedanabdul198
Which statement can best be concluded from the ideal gas law? the product of pressure and volume of an ideal gas is proportional to the absolute temperature. all collisions between atoms or molecules are perfectly elastic and are not the result of any attractive forces. the temperature, pressure, and volume of a gas are all related. the behavior of a gas under real conditions does not obey the ideal gas law.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1
You know the right answer?
Which statement can best be concluded from the ideal gas law? the product of pressure and volume of...
Questions



Mathematics, 25.02.2021 20:20

Mathematics, 25.02.2021 20:20

Spanish, 25.02.2021 20:20

Chemistry, 25.02.2021 20:20

Mathematics, 25.02.2021 20:20


Mathematics, 25.02.2021 20:20

Mathematics, 25.02.2021 20:20

Arts, 25.02.2021 20:20




Mathematics, 25.02.2021 20:20

Mathematics, 25.02.2021 20:20



Mathematics, 25.02.2021 20:20

Mathematics, 25.02.2021 20:20

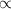 nT
nT

