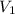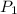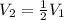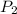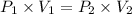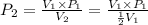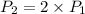Chemistry, 12.01.2020 01:31 middlegirlrule6848
The volume of a gas decreases to half of its original volume, but the gas maintains the same number of moles and temperature. according to the ideal gas law, what will most likely happen to the pressure?
a.)it will double.
b.)it will decrease.
c.)it will increase slightly.
d.)it will remain the same.

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1

Chemistry, 23.06.2019 11:00
Intermolecular forces. question i need with: the only intermolecular forces that affect non polar molecules are forces.
Answers: 2

Chemistry, 23.06.2019 13:30
Explain the impact that changing the temperature has on a system in a state of dynamic equilibrium. what will happen when the temperature of an exothermic reaction mixture at equilibrium is increased?
Answers: 3
You know the right answer?
The volume of a gas decreases to half of its original volume, but the gas maintains the same number...
Questions




Mathematics, 13.02.2021 03:10


Mathematics, 13.02.2021 03:10



History, 13.02.2021 03:10


Mathematics, 13.02.2021 03:10

Mathematics, 13.02.2021 03:10





Mathematics, 13.02.2021 03:10


History, 13.02.2021 03:10

Geography, 13.02.2021 03:10