
Chemistry, 07.12.2019 17:31 xXCoryxKenshinXx
Calculate the mass of chromium metal produced when 425.0ml of 0.25m chromium(ll) nitrate reacts with a strip of zinc that remains in excess. also write and balance an equation.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

You know the right answer?
Calculate the mass of chromium metal produced when 425.0ml of 0.25m chromium(ll) nitrate reacts with...
Questions

Spanish, 06.05.2021 14:00


History, 06.05.2021 14:00


Computers and Technology, 06.05.2021 14:00



Computers and Technology, 06.05.2021 14:00




Mathematics, 06.05.2021 14:00

Social Studies, 06.05.2021 14:00






English, 06.05.2021 14:00

Biology, 06.05.2021 14:00

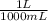 x
x 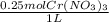 x
x 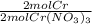 x
x 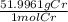 = 5.52 g
= 5.52 g 

