Read the given equation.
2na + 2h2o → 2naoh + h2
during a laboratory experim...

Chemistry, 30.09.2019 08:30 winterblanco
Read the given equation.
2na + 2h2o → 2naoh + h2
during a laboratory experiment, a certain quantity of sodium metal reacted with water to produce sodium hydroxide and hydrogen gas. what was the initial quantity of sodium metal used if 6.30 liters of h2 gas were produced at stp?
a. 10.3 grams
b. 12.9 grams
c. 14.7 grams
d.15.2 grams

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1


Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1
You know the right answer?
Questions

Mathematics, 24.11.2020 22:00

Mathematics, 24.11.2020 22:00

Biology, 24.11.2020 22:00

Mathematics, 24.11.2020 22:00

English, 24.11.2020 22:00


Mathematics, 24.11.2020 22:00

Mathematics, 24.11.2020 22:00








Mathematics, 24.11.2020 22:00




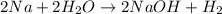
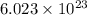 of particles and weighs equal to the molecular mass.
of particles and weighs equal to the molecular mass.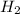 is produced by 46 gram of sodium metal
is produced by 46 gram of sodium metal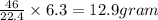 of sodium metal
of sodium metal


