
Chemistry, 04.02.2020 02:01 hackman1216
Which of the following would shift the following equilibrium system to the right? c2h2(g) + h2o(g) ⇌ ch3cho(g) removing h2o(g) from the system adding ch3cho(g) to the system removing c2h2(g) from the system adding h2o(g) to the system

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:50
Where are chemicals found at work? a. only in cleaning products b. only in carpets and paint c. in every area of work d. only in food preparation submit
Answers: 1

Chemistry, 21.06.2019 23:50
2points why do scientists need governmental funding? o a. government politicians ask all the important scientific questions. o b. scientists have to pay taxes to the government on the money they make. o c. the cost of doing scientific research can be very high. o d. the government is controlled by scientists. submit
Answers: 3

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1
You know the right answer?
Which of the following would shift the following equilibrium system to the right? c2h2(g) + h2o(g)...
Questions

History, 12.06.2020 19:57


English, 12.06.2020 19:57

Mathematics, 12.06.2020 19:57

Mathematics, 12.06.2020 19:57




Social Studies, 12.06.2020 19:57



Mathematics, 12.06.2020 19:57








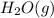 to the system.
to the system.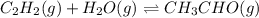
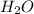 is increased
is increased

