
Chemistry, 06.10.2019 07:11 kberly3750ovgw6f
According to the following balanced equation, 2 formula units of iron (iii) oxide (fe2o3) can be formed by reacting 4 atoms of iron (fe) with 3 molecules of oxygen gas (o2). if 12 atoms of iron are reacted with 6 molecules of oxygen gas, which is the limiting reactant and how many atoms or molecules will be left over? 4fe + 3o2 -> 2fe2o3

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 18:30
Read the claim. breakfast is an important meal. it jump starts the body’s process of using calories to break down food. appetite can decrease with age, but going too long without eating causes metabolism to slow down. current research shows that incorporating legumes such as lentils and chickpeas into meals boosts metabolism for twenty-four hours. who might benefit from this claim? people who have a fast metabolism stores that sell exercise equipment people who take vitamin supplements grocery stores that sell legumes
Answers: 1
You know the right answer?
According to the following balanced equation, 2 formula units of iron (iii) oxide (fe2o3) can be for...
Questions


Mathematics, 14.04.2020 20:30





Mathematics, 14.04.2020 20:31




Biology, 14.04.2020 20:31

Mathematics, 14.04.2020 20:31



Chemistry, 14.04.2020 20:31




Social Studies, 14.04.2020 20:31

Social Studies, 14.04.2020 20:31

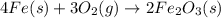
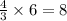 atoms of iron.
atoms of iron.

