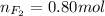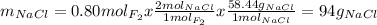Part 1. a chemist reacted 18.0 liters of f2 gas with nacl in the laboratory to form cl2 gas and naf. use the ideal gas law equation to determine the mass of nacl that reacted with f2 at 290. k and 1.5 atm.
f2 + 2nacl → cl2 + 2naf
part 2. explain how you would determine the mass of sodium chloride that can react with the same volume of fluorine gas at stp.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2


Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1
You know the right answer?
Part 1. a chemist reacted 18.0 liters of f2 gas with nacl in the laboratory to form cl2 gas and naf....
Questions


Mathematics, 23.10.2020 18:00

Social Studies, 23.10.2020 18:00

History, 23.10.2020 18:00

English, 23.10.2020 18:00

History, 23.10.2020 18:00

Mathematics, 23.10.2020 18:00

Chemistry, 23.10.2020 18:00


Mathematics, 23.10.2020 18:00

Social Studies, 23.10.2020 18:00


Social Studies, 23.10.2020 18:00

Biology, 23.10.2020 18:00

Mathematics, 23.10.2020 18:00


English, 23.10.2020 18:00

Mathematics, 23.10.2020 18:00


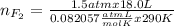 →
→ 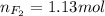
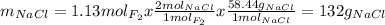
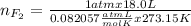 →
→