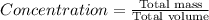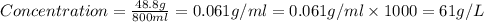Chemistry, 28.09.2019 01:00 trevorhenyan51
Read the statement.
a 200 ml nacl solution with a concentration of 4.0 g/l is mixed with a 600 ml solution containing 8% nacl (m/v).
what is the final concentration of salt in the solution in g/l?
a.4.7
b.61
c.6.1
d.47

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which statements describe polyatomic ions? check all that apply. polyatomic ions have many charges. polyatomic ions have one overall charge. polyatomic ions repel other ions to form ionic bonds. polyatomic ions attract other ions to form ionic bonds. polyatomic ions are made up of only one type of atom. polyatomic ions are made up of two or more types of atoms.
Answers: 2

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2
You know the right answer?
Read the statement.
a 200 ml nacl solution with a concentration of 4.0 g/l is mixed wit...
a 200 ml nacl solution with a concentration of 4.0 g/l is mixed wit...
Questions

History, 02.10.2020 14:01




Mathematics, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01

Biology, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01

History, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01

Arts, 02.10.2020 14:01




English, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01

Medicine, 02.10.2020 14:01


Biology, 02.10.2020 14:01

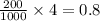 grams of mass of NaCl
grams of mass of NaCl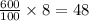 grams of NaCl
grams of NaCl