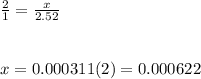Chemistry, 04.02.2020 14:57 alexis3744
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out 28.mg of oxalic acid h2c2o4 , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in 250.ml of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used 28.4ml of sodium hydroxide solution. calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:10
Can smoke be transformed into liquid or used as energy or both?
Answers: 2

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

You know the right answer?
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs ou...
Questions

Mathematics, 29.06.2019 16:00




Mathematics, 29.06.2019 16:00




History, 29.06.2019 16:00







Mathematics, 29.06.2019 16:00

Computers and Technology, 29.06.2019 16:00


History, 29.06.2019 16:00