
Chemistry, 23.11.2019 17:31 BreBreDoeCCx
A410-g cylinder of brass is heated to 95.0*c and placed in a calorimeter containing 335 g of water at 25.0*c. the water is stirred, and its highest temperature is recorded as 32.0*c. from the thermal energy gained by the water, determine the specific heat of brass. the specific heat of water is 4.18 j/g*c.
show your work

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3


Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3
You know the right answer?
A410-g cylinder of brass is heated to 95.0*c and placed in a calorimeter containing 335 g of water a...
Questions

Mathematics, 25.09.2020 19:01



Mathematics, 25.09.2020 19:01



History, 25.09.2020 19:01

History, 25.09.2020 19:01


History, 25.09.2020 19:01

History, 25.09.2020 19:01


English, 25.09.2020 19:01


Mathematics, 25.09.2020 19:01


Mathematics, 25.09.2020 19:01


Mathematics, 25.09.2020 19:01

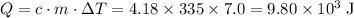 .
.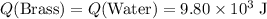 .
.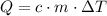 ,
,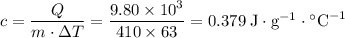 .
.

