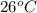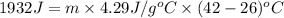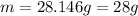Chemistry, 27.09.2019 00:30 kaleighlong959
Asubstance of unknown mass absorbs 1932 joules of energy, going from 26 to 42 degrees celsius. if the specific heat of the substance is 4.29 j/g·°c, how much of the substance is present?
2.8 g
6.6 g
66.2 g
28 g

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3

Chemistry, 23.06.2019 01:40
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1
You know the right answer?
Asubstance of unknown mass absorbs 1932 joules of energy, going from 26 to 42 degrees celsius. if th...
Questions



Computers and Technology, 21.11.2019 01:31

















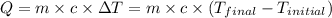
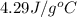
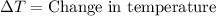
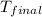 = final temperature =
= final temperature = 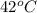
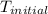 = initial temperature =
= initial temperature =