

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3
You know the right answer?
Read the chemical equation.
n2 + 3h2 → 2nh3
using the volume ratio, determin...
n2 + 3h2 → 2nh3
using the volume ratio, determin...
Questions






Business, 04.11.2020 22:20




Chemistry, 04.11.2020 22:20

Advanced Placement (AP), 04.11.2020 22:20

Physics, 04.11.2020 22:20

Spanish, 04.11.2020 22:20

Mathematics, 04.11.2020 22:20

Arts, 04.11.2020 22:20

Social Studies, 04.11.2020 22:20

English, 04.11.2020 22:20



Advanced Placement (AP), 04.11.2020 22:20

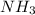 produced is, 0.8 liters
produced is, 0.8 liters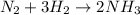
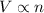 (At constant temperature and pressure)
(At constant temperature and pressure)
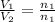
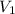 = volume of hydrogen = 1.2 L
= volume of hydrogen = 1.2 L
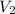 = volume of ammonia = ?
= volume of ammonia = ?
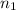 = moles of hydrogen = 3 mole
= moles of hydrogen = 3 mole
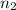 = moles of ammonia = 2 mole
= moles of ammonia = 2 mole
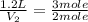
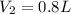
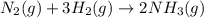
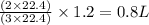 of ammonia
of ammonia

