
Chemistry, 21.11.2019 09:31 rubianny03
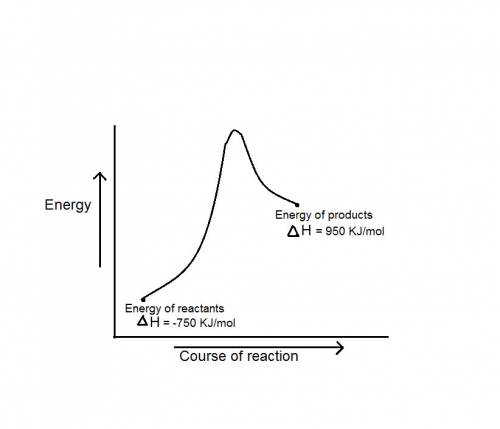
during a reaction, δh for reactants is −750 kj/mol and δh for products is 920 kj/mol. which statement is correct about the reaction?
it is endothermic because the energy required to break bonds in the reactants is less than the energy released when the products are formed.
it is endothermic because the energy required to break bonds in the reactants is greater than the energy released when the products are formed.
it is exothermic because the energy required to break bonds in the reactants is less than the energy released when the products are formed.
it is exothermic because the energy required to break bonds in the reactants is greater than the energy released when the products are formed.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 23.06.2019 04:20
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1

Chemistry, 23.06.2019 07:00
What are the trends and exceptions to the trends in electron affinity?
Answers: 1
You know the right answer?
during a reaction, δh for reactants is −750 kj/mol and δh for products is 920 kj/mol. which statemen...
Questions








Medicine, 18.07.2019 04:20

Computers and Technology, 18.07.2019 04:20

Biology, 18.07.2019 04:20

Engineering, 18.07.2019 04:20

Medicine, 18.07.2019 04:20




Computers and Technology, 18.07.2019 04:20

Computers and Technology, 18.07.2019 04:20


Social Studies, 18.07.2019 04:20

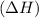 comes out to be positive.
comes out to be positive.

