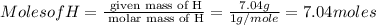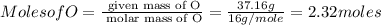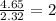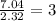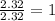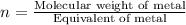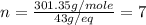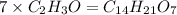Chemistry, 01.10.2019 17:30 manaisialockhart12
Asubstance has 55.80% carbon, 7.04% hydrogen, and 37.16% oxygen. what is it's empirical and molecular formula if it has a molar mass of 301.35 grams

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 23.06.2019 05:00
Scientists discovered fossils in several layers of the earth you see here. they found fossils of algae, snails, and clams in layer d. given that information, where do you think they found fossil evidence of simple land plants and amphibians?
Answers: 1

Chemistry, 23.06.2019 08:30
Kelly has come up with an explanation for why her sister is sometimes in a good mood and other times in a bad mood. she speculates that it is based on the hours of sleep her sister got the previous night. this explanation for her sister's behaviors is an example of a(n)
Answers: 3
You know the right answer?
Asubstance has 55.80% carbon, 7.04% hydrogen, and 37.16% oxygen. what is it's empirical and molecula...
Questions



History, 24.09.2019 00:00

Mathematics, 24.09.2019 00:00

Mathematics, 24.09.2019 00:00

Mathematics, 24.09.2019 00:00

Health, 24.09.2019 00:00

History, 24.09.2019 00:00

Social Studies, 24.09.2019 00:00






Business, 24.09.2019 00:00

History, 24.09.2019 00:00

Mathematics, 24.09.2019 00:00


Biology, 24.09.2019 00:00

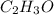 and molecular formula is
and molecular formula is 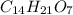
![Moles of C =[tex] \frac{\text{ given mass of C}}{\text{ molar mass of C}}= \frac{55.80g}{12g/mole}=4.65moles](/tpl/images/0280/2916/25d2c.png)