
Chemistry, 29.12.2019 08:31 jmullins3611
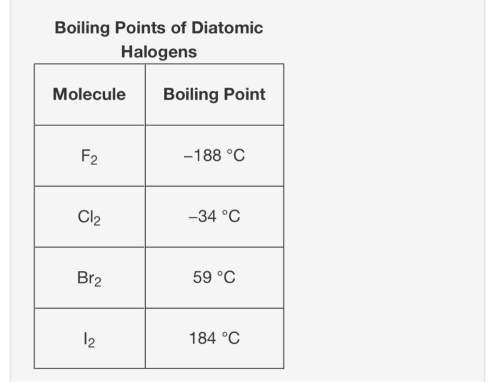
Which of the following statements best explains the trends in boiling points?
a.)the atomic size increases down the group, and this decreases the strength of the intermolecular forces.
b.)the total number of electrons decreases down the group, and this decreases the strength of the intermolecular forces.
c.)the total number of electrons increases down the group, and this increases the strength of the intermolecular forces.
d.)the chances of forming a permanent dipole increases down the group, and this increases the strength of the intermolecular forces.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1
You know the right answer?
Which of the following statements best explains the trends in boiling points?
a.)the at...
a.)the at...
Questions

Mathematics, 08.03.2021 18:00


History, 08.03.2021 18:00

History, 08.03.2021 18:00





Mathematics, 08.03.2021 18:00


Arts, 08.03.2021 18:00



Health, 08.03.2021 18:00



Mathematics, 08.03.2021 18:00





