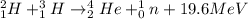The characteristics of two different types of reactions are shown below:
reaction a: an ato...

Chemistry, 21.10.2019 14:30 lindsayb2000
The characteristics of two different types of reactions are shown below:
reaction a: an atom loses electrons during the reaction.
reaction b: an atom loses protons and neutrons during the reaction.
which statement is true about the two reactions?
both reactions retain the identity of the elements.
both reactions change the identity of the elements.
reaction a produces more energy than reaction b.
reaction b produces more energy than reaction a.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
How many atoms are in 1.4 mil of phosphorus trifluoride (pf3)
Answers: 3

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 21:00
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1

Chemistry, 23.06.2019 06:00
What volume of 0.500 mol/l hydrochloric acid, hci (aq) is required to react completely with 1.00 g of aluminum hydroxide, ai(oh)3 (s)?
Answers: 1
You know the right answer?
Questions



Mathematics, 01.07.2021 06:40


English, 01.07.2021 06:40







Mathematics, 01.07.2021 06:50


Mathematics, 01.07.2021 06:50




Mathematics, 01.07.2021 06:50


Arts, 01.07.2021 06:50

 .
.