
Chemistry, 28.10.2019 14:31 hayleegreenwell34
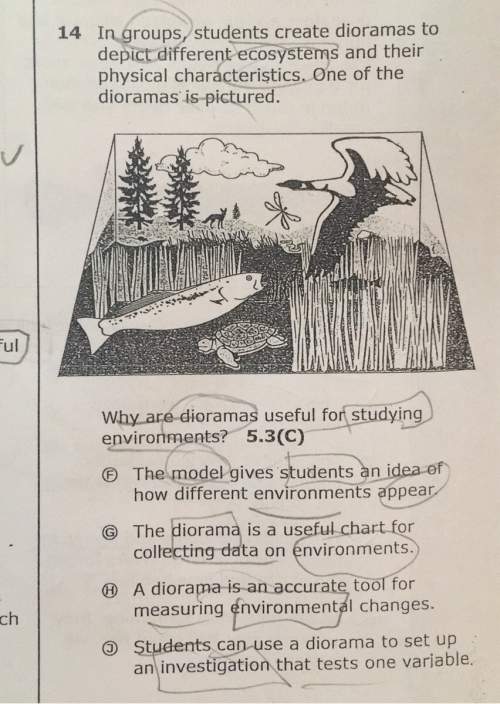
14. in groups, students create dioramas to depict different ecosystems and their physical characteristics. one of the dioramas is pictured why are dioramas useful for studying environments? 5.3(c) e the model gives students an idea of how different environments appear g the diorama is a useful chart for collecting data on environments a is e tool for measuring environmental changes students can use a diorama to set up an investigation that tests one variable

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
What is lincoln's purpose in writing this speech? question 1 options: to stress the difficulties of war to honor those who died in the war to call for an end to the war to call the country to join a new war
Answers: 1

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 08:30
Since the gas in your graduated cylinder is a mixture of butane and water vapor, you must determine the partial pressure of the butane, pbutane, alone. to do this, consult a reference and record the partial pressure of the water vapor, pwater, at the temperature you recorded. use the following formula to compute the partial pressure of the butane. pbutane = atmosphere - pwater use the following combined gas law formula and compute the volume that the butane sample will occupy at stp. (hint: convert both temperatures to kelvin.) pbutane x voriginal = pstandard x vfinal troom tstandard use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. grams of butane you used “x” grams of butane ml of butane corrected to stp = 22,400 ml compute the theoretical molar mass of butane based on its formula and the atomic masses on the periodic table. compare your experimental results from #3 to the theoretical value of #4, computing a percent error of your findings using this formula: % error = measured value - accepted value x 100 accepted value use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. need asap
Answers: 1

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2
You know the right answer?
14. in groups, students create dioramas to depict different ecosystems and their physical characteri...
Questions


Biology, 07.09.2020 14:01


Physics, 07.09.2020 14:01

English, 07.09.2020 14:01

Mathematics, 07.09.2020 14:01



Mathematics, 07.09.2020 14:01

Computers and Technology, 07.09.2020 14:01

Mathematics, 07.09.2020 14:01


Mathematics, 07.09.2020 14:01



Computers and Technology, 07.09.2020 14:01

Mathematics, 07.09.2020 14:01

Mathematics, 07.09.2020 14:01

Mathematics, 07.09.2020 14:01

Spanish, 07.09.2020 14:01



