
Chemistry, 27.01.2020 20:31 issjzjjsmsm
The specific heat capacity (c) of a substance is the thermal energy required
to raise one gram of the substance by one degree celsius. the total amount
of thermal energy (q) added to a sample of a substance can be calculated
by multiplying the specific heat capacity (c) by the mass of the sample (m)
and the temperature change (t): q c m t. it takes 125 kilojoules (kj)
of energy to heat a cup of water, increasing its temperature by 1°c. what
is the temperature change in °c of the cup of water after 750 kj of energy
are used? s

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Select the correct answer. what is the nature of the se-cl bond in a molecule of selenium chloride (secl2) if the electronegativity value of selenium is 2.55 and that of chlorine is 3.16?
Answers: 3

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3
You know the right answer?
The specific heat capacity (c) of a substance is the thermal energy required
to raise one gram...
to raise one gram...
Questions

Mathematics, 11.03.2021 08:30

Computers and Technology, 11.03.2021 08:30


Spanish, 11.03.2021 08:30

Spanish, 11.03.2021 08:30

Mathematics, 11.03.2021 08:30

Mathematics, 11.03.2021 08:30

Mathematics, 11.03.2021 08:30


Spanish, 11.03.2021 08:30

Spanish, 11.03.2021 08:30

Biology, 11.03.2021 08:30




Law, 11.03.2021 08:30


Mathematics, 11.03.2021 08:30


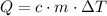 .
. nor
nor 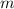 is given.
is given.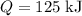 to this cup of water of mass
to this cup of water of mass 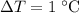 .
.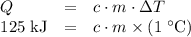
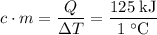 .
.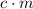 in the second calculation. Here's how:
in the second calculation. Here's how: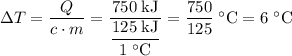 .
.



