
Chemistry, 31.10.2019 16:31 conceitedkayy1865
The following chemical equation shows the incomplete formula for burning methanol. the question mark represents the unknown number of oxygen (o2) molecules. how many molecules of o2 are needed to balance this chemical equation? *
1 point
captionless image
1
3
5
6
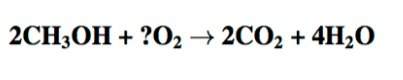

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1

Chemistry, 23.06.2019 05:30
Based on the formulas, select the compounds below that are covalent: kbr sif4 al2o3 co2 naco3 s7o2 pcl3 fe3n2 h2o s2f10
Answers: 3
You know the right answer?
The following chemical equation shows the incomplete formula for burning methanol. the question mark...
Questions

English, 23.11.2020 23:30

Computers and Technology, 23.11.2020 23:30

Mathematics, 23.11.2020 23:30

History, 23.11.2020 23:30


Mathematics, 23.11.2020 23:30


Mathematics, 23.11.2020 23:30




English, 23.11.2020 23:30


Mathematics, 23.11.2020 23:30






Mathematics, 23.11.2020 23:30




