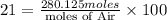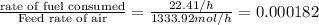Chemistry, 13.10.2019 17:20 alyxkellar06
4. gasoline, which we will represent as having the properties of isooctane, c8h18, is consumed by an idling automobile engine at a rate of 1 gal/h. co is a toxic air pollutant formed from the incomplete combustion of the gasoline. an air monitor in the garage where work is being done on an engine detects an accumulation of co. what does this information imply about the gasoline-to-air ratio being fed to the engine? if we assume that gasoline has properties of isooctane, estimate the feed rate (mol/h) of air for 10% excess oxygen fed to the engine. the mw and density of isooctane is 114.23 g/mol and 691.87 kg/m3, respectively. air contains 21 mol % o2. (hint: balance the stoichiometric equation to determine the theoretical number of moles of o2, c8h18 + n o2 = n h2o + n co2).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:50
2points why do scientists need governmental funding? o a. government politicians ask all the important scientific questions. o b. scientists have to pay taxes to the government on the money they make. o c. the cost of doing scientific research can be very high. o d. the government is controlled by scientists. submit
Answers: 3

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2
You know the right answer?
4. gasoline, which we will represent as having the properties of isooctane, c8h18, is consumed by an...
Questions

Chemistry, 22.10.2020 19:01



Social Studies, 22.10.2020 19:01

Mathematics, 22.10.2020 19:01




History, 22.10.2020 19:01




Physics, 22.10.2020 19:01

Computers and Technology, 22.10.2020 19:01



Mathematics, 22.10.2020 19:01

Health, 22.10.2020 19:01

Mathematics, 22.10.2020 19:01

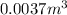
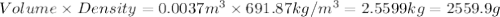
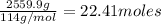
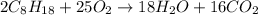
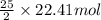 of oxygen that is 280.125 moles.
of oxygen that is 280.125 moles.