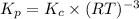Chemistry, 04.02.2020 04:43 kris22elizondop9v1bb
Consider the following equilibrium:
4fe(s) + 3 o2(g) < -- --> 2fe2o3(s);
which of the following equations is wrong?
kp=kc(rt)-5
kc=[o2]-3
kp= kc(rt)-3
kp = po2-3

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
You know the right answer?
Consider the following equilibrium:
4fe(s) + 3 o2(g) < -- --> 2fe2o3(s);
<...
4fe(s) + 3 o2(g) < -- --> 2fe2o3(s);
<...
Questions


English, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00

Biology, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00

History, 13.03.2021 01:00

History, 13.03.2021 01:00


Mathematics, 13.03.2021 01:00




Mathematics, 13.03.2021 01:00





Mathematics, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00

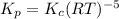
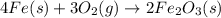
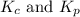 is given by:
is given by:![K_c=\frac{1}{[O_2]^3}](/tpl/images/0499/0672/4f5e0.png)
![K_p=\frac{1}{[O_2]^3}](/tpl/images/0499/0672/d3f6c.png)
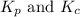 is given by the expression:
is given by the expression: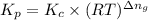
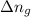 = number of moles of gaseous products - number of moles of gaseous reactants
= number of moles of gaseous products - number of moles of gaseous reactants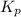 is:
is: