

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

You know the right answer?
Calculate the mass (g) of agcl formed when 174 g of silver sulfide reacts with excess hydrochloric a...
Questions



English, 02.12.2020 02:00



Medicine, 02.12.2020 02:00


Mathematics, 02.12.2020 02:00

English, 02.12.2020 02:00

Mathematics, 02.12.2020 02:00

Mathematics, 02.12.2020 02:00

History, 02.12.2020 02:00


Mathematics, 02.12.2020 02:00

Mathematics, 02.12.2020 02:00


English, 02.12.2020 02:00

Mathematics, 02.12.2020 02:00

Mathematics, 02.12.2020 02:00

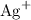 , andSulfide ions
, andSulfide ions 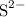 .
.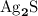 .
.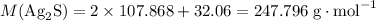 .
.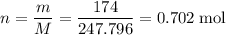 .
. .
.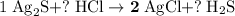 .
.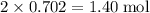 of AgCl.
of AgCl.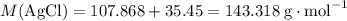 .
.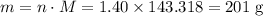 .
.

