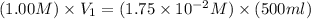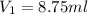Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

You know the right answer?
What volume of a 1.00 m stock solution of glucose must be used to make 500.0 ml of a 1.75 ✕ 10–2 m g...
Questions

Mathematics, 06.04.2021 07:10


Mathematics, 06.04.2021 07:10

Mathematics, 06.04.2021 07:10


Mathematics, 06.04.2021 07:10




Mathematics, 06.04.2021 07:20



Computers and Technology, 06.04.2021 07:20

Mathematics, 06.04.2021 07:20


Biology, 06.04.2021 07:20


Social Studies, 06.04.2021 07:20


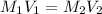
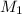 = molarity of stock solution = 1.00 M
= molarity of stock solution = 1.00 M
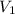 = volume of stock solution = ?
= volume of stock solution = ?
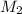 = molarity of glucose solution =
= molarity of glucose solution = 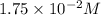
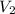 = volume of glucose solution = 500 ml
= volume of glucose solution = 500 ml