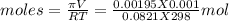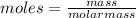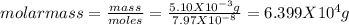Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1
You know the right answer?
Asolution was made by dissolving 5.10 mg of hemoglobin in water to give a final volume of 1.00 ml. t...
Questions





Mathematics, 04.12.2020 01:00


Mathematics, 04.12.2020 01:00

English, 04.12.2020 01:00


Mathematics, 04.12.2020 01:00

Mathematics, 04.12.2020 01:00




Mathematics, 04.12.2020 01:00


Biology, 04.12.2020 01:00

Mathematics, 04.12.2020 01:00


Advanced Placement (AP), 04.12.2020 01:00