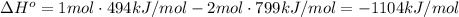Chemistry, 31.10.2019 11:31 24hudsonmoss
Given: c + o2 → co2 bond bond energy (kj/mol) c=o 799 o=o 494 calculate the enthalpy change for the chemical reaction. the change in enthalpy for the given reaction is kilojoules.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 23.06.2019 02:00
Anitrogen atom and an oxygen atom combine chemically to form nitric oxide. what is nitric oxide?
Answers: 1

Chemistry, 23.06.2019 02:30
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1

Chemistry, 23.06.2019 06:00
Robert leaves a chocolate bar in his car while attending school all day. when he goes to his car in the afternoon, the bat has changed into gooey liquid. what happened to the chocolate bar
Answers: 1
You know the right answer?
Given: c + o2 → co2 bond bond energy (kj/mol) c=o 799 o=o 494 calculate the enthalpy change for the...
Questions



Spanish, 20.09.2019 09:10

History, 20.09.2019 09:10

History, 20.09.2019 09:10

Mathematics, 20.09.2019 09:10



History, 20.09.2019 09:10







Biology, 20.09.2019 09:10


Computers and Technology, 20.09.2019 09:10

Geography, 20.09.2019 09:10

Advanced Placement (AP), 20.09.2019 09:10