
Chemistry, 25.09.2019 01:00 genyjoannerubiera
In part iii, the phenolphthalein indicator is used to monitor the equilibrium shifts of the ammonia/ammonium ion system. the phenolphthalein equilibrium established with water is hph(aq)(colorless) + h2o (l) h3o+ (aq) + ph-(aq)(pink or red). you compared the color of the solutions in three test tubes that initially contained 3 ml of 0.1 m ammonium hydroxide and a few drops of phenolphthalein indicator. in the first test tube, you added 1 m nh4cl dropwise. what color change was observed and what did this color change indicate about the shift in the phenolphthalein equilibrium? a. the solution turned a more intense pink or red color indicating that the phenolphthalein equilibrium shifted to the left, producing more of the pink or red colored hph.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1
You know the right answer?
In part iii, the phenolphthalein indicator is used to monitor the equilibrium shifts of the ammonia/...
Questions

Mathematics, 10.12.2020 19:50



Mathematics, 10.12.2020 19:50

English, 10.12.2020 19:50


Mathematics, 10.12.2020 19:50




Biology, 10.12.2020 19:50

Health, 10.12.2020 19:50

Mathematics, 10.12.2020 19:50



Mathematics, 10.12.2020 19:50



Mathematics, 10.12.2020 19:50

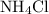 is a salt soluble in water.
is a salt soluble in water. 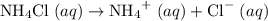 .
.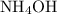 .
. 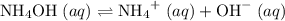 .
.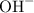 and
and 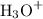 ions.
ions.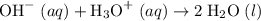 .
.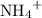 ions in the solution. Some of the
ions in the solution. Some of the 

